
Alcohols are the compounds of the general formula R – OH, where R is an alkyl or a substituted alkyl group. Thus, alcohols are the derivatives of alkanes in which one or more H atoms are replaced by -OH.

Alcohols are very important industrial chemicals. Ethanol is widely used as an antiseptic in the form of a rectified spirit. It is the main component of all alcoholic beverages and is used as a solvent for lacquers and varnishes.
Alcohol can be prepared by general methods as well as by industrial processes.
Haloalkanes, when heated with aqueous alkali, or moist silver oxide, gives the corresponding monohydric alcohols.

Slow decomposition of large organic molecules (carbohydrates, sugar and starch) in the presence of suitable enzymes produces alcohol. This method can be used to prepare ethanol by fermenting glucose.

The physical and chemical properties of alcohols are primarily due to the presence of a hydroxyl group.
Alcohols exhibit a wide range of spontaneous chemical reactions due to the cleavage of the C-O bond and O-H bond. Some prominent chemical reactions of alcohol are:
Oxidation Reaction – Alcohols are oxidised in the presence of an oxidising agent to produce aldehydes and ketones, which can then be oxidised further to produce carboxylic acids.

Reaction with metals – Alcohols react with active metals such as sodium, potassium etc. to form the corresponding alkoxide.

Dehydration of Alcohols – When exposed to an acidic medium, alcohols dehydrate, which results in the formation of an alkene.

Esterification – When alcohol reacts with a carboxylic acid in the presence of a catalyst, it results in the formation of a sweet-smelling compound called an ester.

Alcohols can be classified on the following basis:
i. On the basis of the number of -OH groups present in the molecule.
ii. On the basis of the nature of the carbon atom bonded to the -OH group.

Alcohols are classified into three types- Primary, Secondary, and Tertiary based on the number of carbon atoms directly attached to the carbon bonded with the -OH group.


Alcohols are organic molecules with a functional hydroxyl or –OH group bonded directly to carbon. Alcohol gets its name from the hydrocarbon from which it is derived.
There are three systems of naming alcohols-
The common name of alcohol can be obtained by adding the word alcohol after the alkyl group. Alternatively, the common name of alcohol can be obtained by replacing the ending -ane of the parent hydrocarbon by yl, and adding the word alcohol thereafter.
For alcohols containing three or more carbon atoms, there can be more than one location of the -OH group. Such alcohols are named primary, secondary or tertiary alcohols. They are often named using the prefixes as n or iso-.

In this system, the name of alcohol is obtained by replacing the last ‘e’ in the name of the parent alkane, akene or alkyne by the suffix ‘ol’. However, the higher and branched-chain compounds follow certain procedures.
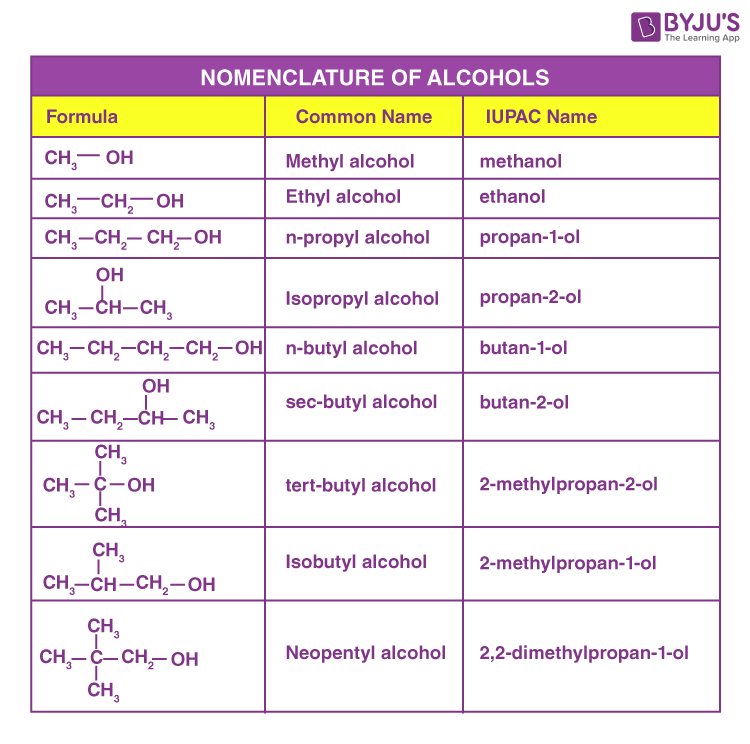
Some of the commonly used alcohols are methanol, ethanol, propanol and butanol.